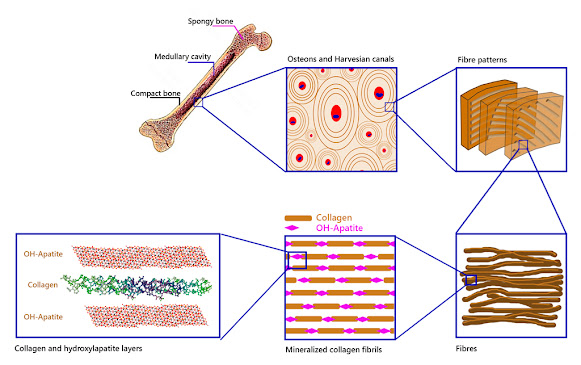
The endoskeleton of many animals is made up of rigid, solid, and resistant organs which are the bones. Bones, despite their inert appearance, are alive and contain cells, blood vessels, nerves, fat, bone marrow. They are in constant transformation. Bone tissue is the basis of its structure. Bone cells are only 2% of bone tissue, the rest is the extracellular matrix. This matrix is made up of 30% organic substances such as the protein called collagen. The remaining 70% is an inorganic substance: hydroxylapatite.
The solidity of the bone tissue is given by the presence of hydroxylapatite crystals that are arranged around the collagen fibres forming an important framework, giving rise to the exceptional mechanical properties of bones (figure 1). A fairly accurate comparison would be the structure of a building, where the collagen would correspond to the steel rods and the hydroxylapatite crystals to the concrete that surrounds them (UCM, online).
But it is not only in the bones that hydroxylapatite can be found. Although bones seem very strong, the hardest substance in our body is tooth enamel, which is also made up of hydroxylapatite.
As it can be seen, OH-apatite is very present in the organic world, the world of living beings. But it can also be found in nature, in the inorganic world, in the mineral kingdom.
T= P⁵⁺, As⁵⁺, V⁵⁺, Si⁴⁺, S⁶⁺, B³⁺
M1 and M2 are represented by numerous cations in the periodic table (in two different positions, 1 and 2) including the rare earth elements (REE): Ca²⁺, Pb²⁺, Ba²⁺, Sr²⁺, Mn²⁺, Na⁺, Ce³⁺, La³⁺, Y³⁺, Bi³⁺.
And to complete the formula, X would correspond to: (OH)⁻, F⁻ and/or Cl⁻.
Members of the apatite supergroup, about 50 species, show a wide range of solid solutions due to the substitution of the forming atoms. This supergroup is currently divided into five divisions: apatite group, hedyphane group, belovite group, britholite group, ellestadite group.
The one of interest in this note is the group of apatite, which includes several species between phosphates, arsenates, and vanadates, some well-known as pyromorphite, mimetite, or vanadinite, all of them from the hexagonal system (figures 2, 3, and 4).
Figure 2 - Mimetite crystal with cerussite and calcite.
Calcium phosphates are found in this group, which have a simplified formula according to Ca₅(PO₄)₃X
where X = F (fluorapatite), Cl (chlorapatite), OH (hydroxylapatite).
They are often colloquially called "apatite". In nature, the most common are fluorapatite and hydroxylapatite.
Geologically, members of this supergroup are found in different types of igneous, sedimentary, and metamorphic rocks. Even in meteorites (ordinary chondrites L) or in the basaltic breach in Martian meteorites (impactites).
“Apatite”: where does it come from?
The specimens in the photographs correspond to two classic Catalan deposits: Turó de Montcada (Montcada i Reixac) and the Rocabruna mines (Bruguera, Gavà), both in the province of Barcelona. In Catalonia, there are other deposits with similar geology, more or less rich in phosphates, such as Torroja del Priorat, Cornudella de Montsant, Figuerola del Camp, Santa Creu d'Olorda or Pineda de Mar, among others.
They are located mainly in the Catalan Coastal Ranges, which run parallel to the coast, from the south of the province of Tarragona to Girona.
These deposits of stratiform sedimentary origin share a similar origin and are found in materials from the Paleozoic series: shales, limestones, dolomites, and quartzites.
In these deposits, Silurian shales (about 420 million years BP) contain rich levels of marine sedimentary phosphates (phosphorites). When meteoric fluids pass through these shales with pyrite (FeS2), they become acidic (form sulphuric acid) and dissolve them. These fluids, rich in phosphorus, reach areas where carbonate rocks predominate, producing the neutralization of acidity and the precipitation of various minerals, including fluorapatite and hydroxylapatite.
Figure 3 - Divergent and globular aggregates of acicular crystals, on a matrix with goethite and mitridatite.
Figure 4 - Divergent aggregates of acicular crystals, on a matrix with goethite and mitridatite (RM3430).
Figure 5 - Divergent aggregate of acicular crystals, on a matrix with goethite and mitridatite (RM3430).
It should be noted that many of the "apatite" formed by these processes incorporate in their structure carbonate anions (CO₃²⁻) which replace the phosphate anions (PO₄³⁻).
It follows that the varieties carbonate-rich fluorapatite and carbonate-rich hydroxylapatite are present: Ca₅(PO₄,CO₃)₃(F, OH).
This is the case with the specimens in figures 3, 4, and 5.
Figure 6 - SEM image of the specimen from the Turó de Montcada (RM3430).
Differentiating whether it is fluorapatite or hydroxylapatite requires a chemical analysis, with scanning electron microscopy being an ally that is sometimes not very sensitive. In the specimens studied in these two Catalan mines, the presence of both species has been confirmed, but distinguishing those that have fluorine from those that contain hydroxyl groups is not an easy task and impossible with the naked eye (figures 6 and 7).
Figure 7 - SEM-EDS of the specimen from Turó de Montcada (RM3430). The presence of fluorine is not observed in any of the studied points of this specimen. The hexagonal shape of the needles can be observed.
Nature uses the elements at its disposal to form both organic and inorganic structures, mixing both worlds. In this respect, human beings are part of both.
References
Nair, A.K., Gautieri, A., Chang, S.W., Buehler, M.J. (2013): “Molecular mechanics of mineralized collagen fibrils in bone”. Nature Commun. 4, 1724.
Pasero, M., Kampf, A.R., Ferraris, C., Pekov, I.V., Rakovan, J., White, T.J. (2010): “Nomenclature of the apatite supergroup minerals”. European Journal of Mineralogy, 22, 163-179. https://www.researchgate.net/publication/228477092 [on-line 22 Febrero 2022].
Wang, W., Yeung, K.W.K. (2017): “Bone grafts and biomaterials substitutes for bone defect repair: A review”. Bioactive Materials, 2, 224-247. https://www.researchgate.net/publication/317392938 [on-line 22 febr. 2022].
[web] Fisiopatología ósea. Universidad Complutense de Madrid. https://www.ucm.es/data/cont/docs/420-2014-02-18-01%20fisiopatologia%20osea.pdf [on-line 22 febrero 2022].







No comments:
Post a Comment